|
PROFILE.
 This blog is proudly run by two girls : - Nur Rashilah & - Tan Him Gee of MB0801, Nanyang Polytechnic ; School of Chemical & Life Sciences :) CONTENTS. - Formal Welcome - First Scoop To Virology - Viral Replication Strategies - Viral Replication Animation - Viral Genetics - Viroids & Prions - Virusoids - Baltimore Classification CREDITS. linkone linktwo Layout: hearteditorials Codes: -ambulance Icon: biconcave |
Viral Genetics.
Following our topic on viral replication strategies previously, we'll now touch on virus genetics. Viruses grow rapidly, there are usually a large number of progeny virions per cell. There is, therefore, more chance of mutations occurring over a short time period. The nature of the viral genome, be it RNA or DNA, segmented or non-segmented, plays an important role in the genetics of the virus. Moreover, viruses may change genetically due to mutation or recombination. MUTANTS a) Origin Spontaneous mutations These arise naturally during viral replication: e.g. due to errors by the genome-replicating polymerase or a a result of the incorporation of tautomeric forms of the bases DNA viruses tend to more genetically stable than RNA viruses. There are error correction mechanisms in the host cell for DNA repair, but probably not for RNA. Some RNA viruses are remarkably invariant in nature. These viruses probably have the same high mutation rate as other RNA viruses, but are so precisely adapted for transmission and replication that fairly minor changes result in failure to compete successfully with parental (wild-type, wt) virus. Mutations that are induced by physical or chemical means: Chemical: - Agents acting directly on bases, e.g. nitrous acid. - Agents acting indirectly, e.g. base analogs which mispair more frequently than normal bases thus generating mutations. Physical: Agents such as UV light or X-rays. b) Types of mutation Mutants can be point mutants (one base replaced by another) or insertion/deletion mutants. c) Examples of the kinds of phenotypic changes seen in virus mutants. (phenotype = the observed properties of an organism) Conditional lethal mutants: These mutants multiply under some conditions but not others (whereas the wild-type virus grows under both sets of conditions) e.g: temperature sensitive (ts) mutants - These will grow at low temperature. Such as 31 degrees C but not at 39 degrees C, wild type grows at 31 and 39 degrees C. The reason for this is often that the altered protein cannot maintain a functional conformation at the elevated temperature. e.g: host range - These mutants will only grow in a subset of the cell types in which the wild type virus will grow - such mutants provide a means to investigate the role of the host cell in viral infection. Plaque size: Plaques may be larger or smaller than in the wild type virus, sometimes such mutants show altered pathogenicity. Drug resistance: This is important in the development of antiviral agents - the possibility of drug resistant mutants arising must always be considered. Enzyme-deficient mutants: Some viral enzymes are not always essential and so we can isolate viable enzyme-deficient mutants. For example, herpes simplex virus thymidine kinase is usually not required in tissue culture but it is important in infection of neuronal cells. "Hot" mutants: These grow better at elevated temperatures than the wild type virus. They may be more virulent since host fever may have little effect on the mutants but may slow down the replication of wild type virions. Attenuated mutants: Many viral mutants cause much milder symptoms (or no symptoms) compared to the parental virus. These are said to be attenuated and have a potential role in vaccine development. They are also useful tools in determining why the parental virus is harmful. EXCHANGE OF GENETIC MATERIAL Recombination This refers to an exchange of genetic information between two genomes. "Classic" recombination This involves breaking of covalent bonds within the nucleic acid, exchange of genetic information, and reforming of covalent bonds. This kind of break/join recombination is common in DNA viruses or those RNA viruses which have a DNA phase (retroviruses). The host cell has recombination systems for DNA. Recombination of this type is very rare in RNA viruses as there are probably no host enzymes for RNA recombination. Picornaviruses show a form of very low efficiency recombination. The mechanism is not identical to the standard DNA mechanism, and is probably a "copy choice" kind of mechanism in which the polymerase switches templates while copying the RNA.  Recombination is also common in the coronaviruses - again the mechanism is different from the situation with DNA and probably is a consequence of the unusual way in which RNA is synthesized in this virus. a) Mapping genomes (the further apart two genes are, the more likely it is that there will be a recombination event between them).
Recombination enables a virus to pick up genetic information from viruses of the same type and occasionally from unrelated viruses or even the host genome. Reassortment If a virus has a segmented genome and if two variants of that virus infect a single cell, progeny virions can result with some segments from one parent, some from the other. This is an efficient process but is limited to viruses with segmented genomes - so far the only human viruses characterized with segmented genomes are RNA viruses. Some examples include orthomyxoviruses, reoviruses, arenaviruses, bunya viruses. Reassortment may play an important role in nature in generating novel reassortants and has also been useful in laboratory experiments.  http://pathmicro.med.sc.edu/mhunt/gen3.jpg http://pathmicro.med.sc.edu/mhunt/gen3.jpg Reassortment is a non-classical kind of recombination Applied genetics There is vaccine called Flumist (LAIV, approved June 2003) for influenza virus which involves some of the principles discussed above. The vaccine is trivalent – it contains 3 strains of influenza virus:  Antibodies to the influenza virus surface proteins (HA - hemagglutinin and NA - neuraminidase) are important in protection against infection. The HA and NA change from year to year. The vaccine technology uses reassortment to generate reassortant viruses which have six gene segments from the attenuated, cold-adapted virus and the HA and NA coding segments from the virus which is likely to be a problem in the up-coming influenza season. This vaccine is a live vaccine and is given intranasally as a spray and can induce mucosal and systemic immunity. Complementation Multiplicity reactivation Defective viruses 
We can also get the situation where a coat is entirely that of another virus, e.g. a retrovirus nucleocapsid in a rhabdovirus envelope. This kind of phenotypic mixing is sometimes referred to as pseudotype (pseudovirion) formation. The pseudotype described above will show the adsorption-penetration-surface antigenicity characteristics of the rhabdovirus and will then, upon infection, behave as a retrovirus and produce progeny retroviruses. This results in pseudotypes having an altered host range/tissue tropism on a temporary basis. 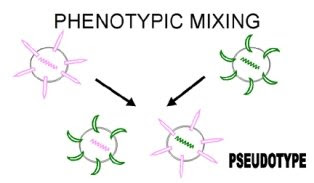 http://pathmicro.med.sc.edu/mhunt/gen6.jpg With that, we have now conclude today's topic on viral genetics. Next up will be on Viroids & Prions. So stay tuned! |
